Are all liquids able to conduct an electric current? Dive into the fascinating world of liquid conductivity and discover the electrifying truth behind this intriguing question. From understanding the concept of electrical conductivity in liquids to exploring the influence of solutes, this comprehensive guide will illuminate the mysteries surrounding the ability of liquids to carry an electric charge.
Not all liquids are created equal when it comes to electricity. While some liquids, like saltwater, can conduct an electric current, others, like oil, are insulators. This is because the ability of a liquid to conduct electricity depends on the presence of free ions, which are atoms or molecules that have lost or gained electrons.
The more free ions a liquid contains, the better it will conduct electricity. An electric utility company determines the monthly bill for electricity based on the amount of electricity used by the customer. The more electricity used, the higher the bill.
This is because electricity is a commodity that must be produced and delivered to customers. The cost of producing and delivering electricity is passed on to customers in the form of their monthly bill.
Prepare to be amazed as we delve into the types of liquids and their conductivity, uncovering the secrets behind conductors, insulators, and semiconductors. Witness the transformative power of dissolved solutes and unravel the concepts of electrolytes and non-electrolytes. But hold on tight, because we’re not just exploring theory; we’re also uncovering the practical applications of liquid conductivity that shape our everyday lives.
It’s not all about how well liquids conduct electricity, but also about how they can be used to power things like electric motors. For example, an electric motor turns a flywheel through the use of liquid electrolytes. This shows that even though not all liquids can conduct electricity, those that can have the potential to be used in a variety of applications.
Conductivity of Liquids
In the realm of electricity, liquids play a crucial role in the transfer of electric currents. The ability of a liquid to conduct electricity, known as its conductivity, is a fascinating property that depends on various factors. Understanding liquid conductivity is essential for numerous applications, ranging from electrochemical devices to industrial processes.
You might be wondering if all liquids can conduct electricity. Well, it turns out that not all liquids are created equal. Some liquids, like salt water, can conduct electricity, while others, like oil, cannot. This is because liquids that conduct electricity contain ions, which are atoms or molecules that have lost or gained electrons.
These ions can move freely through the liquid, allowing electricity to flow. On the other hand, liquids that do not conduct electricity do not contain ions, so electricity cannot flow through them. An electric motor rotating a workshop grinding wheel at high speeds is a good example of how electricity can be used to power a mechanical device.
The motor uses electricity to create a magnetic field, which then causes the grinding wheel to rotate. So, next time you’re wondering if a liquid can conduct electricity, just remember that it all depends on whether or not the liquid contains ions.
The conductivity of a liquid is determined by the presence of free ions or charged particles that can move within the liquid. These ions can originate from dissolved salts, acids, or bases, and their mobility determines the liquid’s ability to conduct electricity.
Yo, let’s talk about liquids and electricity. Not all liquids are down to conduct an electric current, it depends on what’s dissolved in them. Take an electric scooter, for instance, it’s got a battery capable of supplying enough juice to power its motor.
But if you drop that scooter in a pool of pure water, nothing’s gonna happen ’cause water alone can’t carry the electric party.
Types of Liquids and Conductivity
Liquids can be classified into three categories based on their conductivity:
- Conductors:Liquids with a high concentration of free ions, allowing for efficient conduction of electricity. Examples include saltwater and molten metals.
- Insulators:Liquids with a low concentration of free ions, making them poor conductors of electricity. Examples include pure water and non-polar organic solvents.
- Semiconductors:Liquids with an intermediate conductivity, exhibiting properties of both conductors and insulators. Examples include liquid crystals and certain ionic liquids.
Influence of Solutes, Are all liquids able to conduct an electric current
The conductivity of a liquid is heavily influenced by the presence of dissolved solutes. Electrolytes, such as salts, acids, and bases, dissociate into ions when dissolved in water, increasing the number of free ions and enhancing conductivity. Non-electrolytes, on the other hand, do not dissociate into ions and have minimal impact on conductivity.
Not all liquids can conduct electricity, but some, like saltwater, can. In fact, an electrochemical cell that generates electricity contains a liquid electrolyte that conducts ions between the electrodes. This allows the cell to produce an electric current.
Applications of Liquid Conductivity
Liquid conductivity finds numerous practical applications in various industries and devices:
- Electrochemical cells:Batteries and fuel cells rely on the conductivity of electrolytes to facilitate the flow of ions.
- Conductivity sensors:Used to measure the purity of water, detect contaminants, and monitor industrial processes.
- Electroplating:Electrolytes are used to deposit metal coatings on surfaces.
- Medical applications:Conductivity measurements are used in blood analysis, electrophoresis, and other diagnostic techniques.
Limitations of Liquid Conductivity
Despite their wide applications, liquids face certain limitations as conductors:
- Electrolysis:High currents passing through liquids can cause electrolysis, breaking down the liquid and potentially forming gases.
- Polarity:Some liquids, such as water, are polar and can dissolve certain solutes, while others are non-polar and have limited solubility.
- Temperature dependence:Conductivity can vary with temperature, affecting the performance of liquid-based devices.
Closing Notes: Are All Liquids Able To Conduct An Electric Current
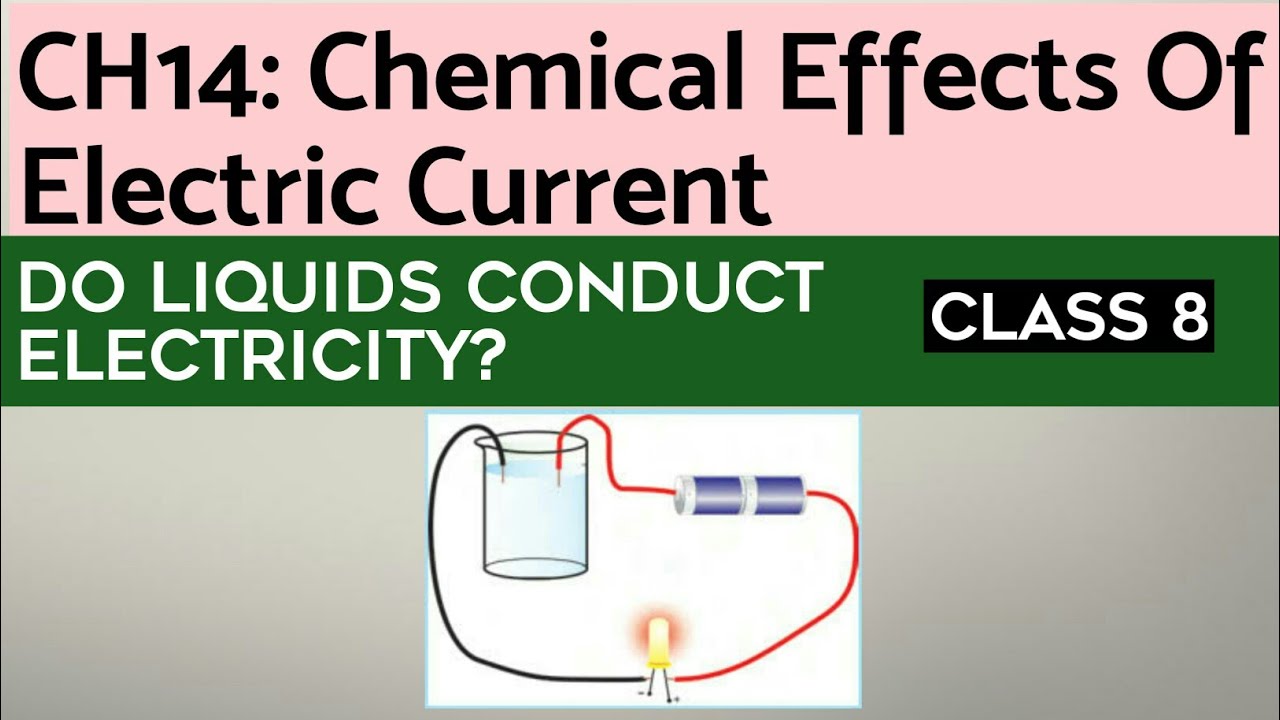
As we reach the end of our electrifying journey, remember that the ability of liquids to conduct electricity is a captivating phenomenon with far-reaching implications. From powering our devices to shaping industrial processes, liquid conductivity plays a vital role in our modern world.
However, it’s important to acknowledge the limitations and challenges associated with using liquids as conductors, ensuring that we harness their potential safely and effectively.
FAQ
What factors influence the conductivity of liquids?
The conductivity of liquids is influenced by factors such as temperature, concentration of ions, and the presence of impurities.
Can all liquids conduct electricity?
No, not all liquids can conduct electricity. Liquids that do not contain ions, such as pure water, are poor conductors of electricity.
What are electrolytes?
Electrolytes are substances that, when dissolved in a liquid, produce ions and increase the conductivity of the liquid.
While not all liquids can conduct electricity, those that do, like saltwater, play a crucial role in the inner workings of an electric motor. An electric motor consists of two electromagnets, one stationary and the other rotating. These magnets interact with each other, creating a force that causes the motor to spin.
The electricity flowing through the liquid conductor is what makes this interaction possible.
Are all liquids able to conduct an electric current? The answer is no. In fact, an electric motor draws 150 amperes , which means that it requires a lot of electrical current to operate. This is because the motor’s windings are made of copper, which is a good conductor of electricity.
However, not all liquids are good conductors of electricity. For example, water is a poor conductor of electricity, which is why it is used in electrical circuits to prevent electrical fires.
Not all liquids can conduct electricity, but some can, like saltwater. This is because liquids with ions in them can carry an electric current. An electric motor may give noise due to the flow of electricity through its windings. This can be caused by a number of factors, such as the type of motor, the load on the motor, and the speed of the motor.
Not all liquids can conduct electricity, but an electric kettle certainly can! An electric kettle uses electricity to heat water, and it does so by passing an electric current through the water. This causes the water to heat up and eventually boil.
So, while not all liquids can conduct electricity, an electric kettle is one that definitely can!