An electric cell is a source of electric current because it undergoes fascinating chemical reactions that generate a flow of electrons. Within its structure, the anode and cathode play crucial roles, facilitating the movement of ions and electrons, ultimately producing the electric current that powers our devices.
Electric cells find widespread applications in our daily lives, from powering flashlights to operating complex electronic gadgets. Understanding their inner workings allows us to appreciate the remarkable technology that shapes our modern world.
An electric cell is a source of electric current because it contains chemicals that react to produce electrons. These electrons can then flow through a circuit, creating an electric current. In a similar vein, adding an electrical outlet from a light switch adding an electrical outlet from a light switch involves creating a new path for electrons to flow, allowing you to power additional devices in your home.
Ultimately, both an electric cell and an electrical outlet rely on the flow of electrons to function, making them essential components of our modern electrical systems.
An Electric Cell: A Powerhouse of Electric Current
In the realm of electrical engineering, an electric cell stands as a cornerstone, providing a steady stream of electric current to power our daily lives. These compact sources of energy harness chemical reactions to generate electricity, making them essential components in a wide range of devices, from tiny watches to colossal industrial machinery.
The Electrochemical Process in an Electric Cell
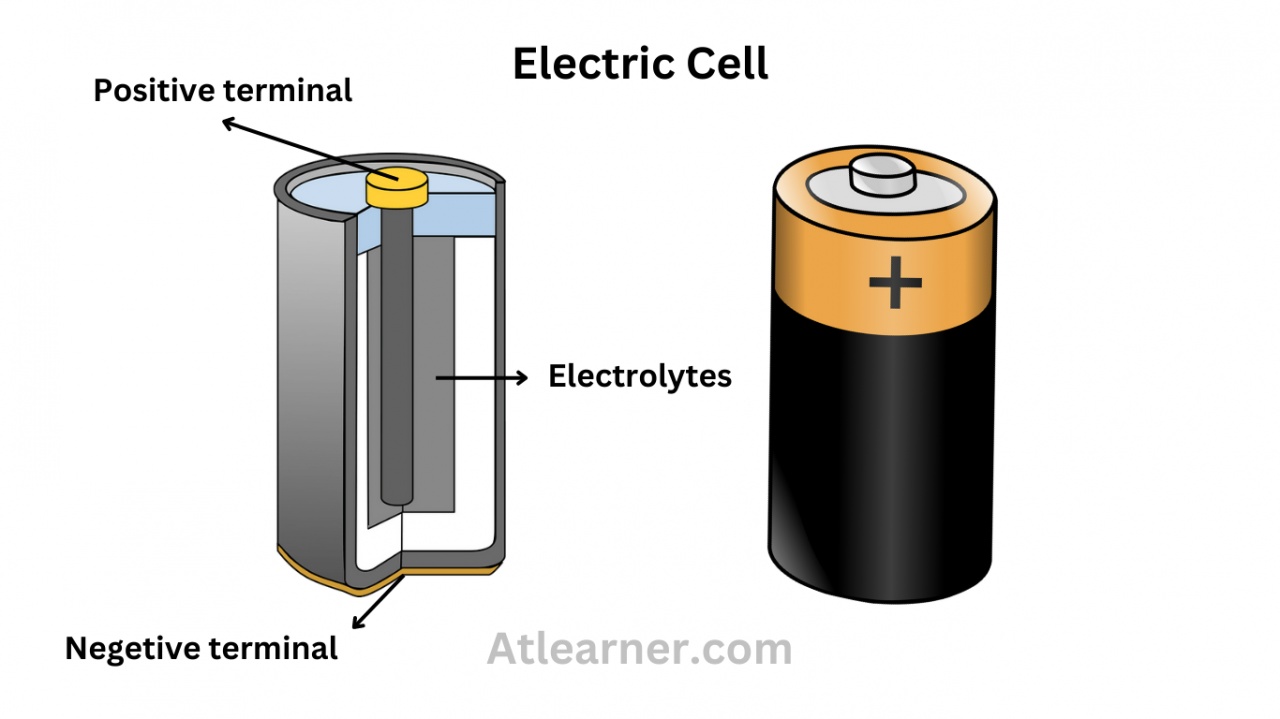
At the heart of an electric cell lies the electrochemical process, a fascinating dance of chemical reactions that ultimately produces electric current. The cell comprises two electrodes, the anode and cathode, immersed in an electrolyte solution. The anode, the site of oxidation, undergoes a chemical reaction where electrons are released.
An electric cell is a source of electric current because it contains chemicals that can produce electrons. These electrons are attracted to the positive terminal of the cell, and they flow through the circuit to the negative terminal. This flow of electrons creates an electric current.
The ability to hold an electrical charge is essential for an electric cell to function. Without this ability, the electrons would not be able to flow through the circuit, and the cell would not be able to produce an electric current.
Click here to learn more about the ability to hold an electrical charge.
These liberated electrons embark on a journey through an external circuit, creating an electric current. Simultaneously, at the cathode, the site of reduction, electrons are accepted, completing the circuit.
An electric cell is a source of electric current because it contains chemicals that react to produce electrons. These electrons flow through a circuit, creating an electric current. Back in the day, electric fences weren’t a thing ( 50 years ago that wasn’t an electric fence ). But nowadays, electric cells power all sorts of devices, from our phones to our cars.
So, there you have it – electric cells are pretty important!
Common electrochemical reactions employed in electric cells include the Daniell cell, which utilizes copper and zinc electrodes in a copper sulfate solution, and the lead-acid battery, a mainstay in automotive applications.
The Role of Electrodes in an Electric Cell
The anode and cathode, the two electrodes in an electric cell, play distinct roles in the electrochemical process. The anode, the electron donor, is typically made of a metal that undergoes oxidation easily, such as zinc or lead. Conversely, the cathode, the electron acceptor, is often composed of a metal that resists oxidation, such as copper or carbon.
An electric cell is a source of electric current because it has a chemical reaction that creates a flow of electrons. However, owning an electric car can come with hidden costs, such as 10 hidden costs of owning an electric car . But despite these costs, an electric cell remains a reliable source of electric current.
The Generation of Electric Current in an Electric Cell, An electric cell is a source of electric current because
The flow of electrons in an electric cell is the driving force behind the generation of electric current. As electrons are released at the anode, they embark on a journey through an external circuit, eager to reunite with their counterparts at the cathode.
An electric cell is a source of electric current because it can generate a flow of electrons. This is achieved through chemical reactions that occur within the cell. The flow of electrons can be used to power various electronic devices.
For instance, a manufacturer uses electrical fuses in an electronic system to protect against short circuits. An electric cell is thus an essential component in many electronic devices, providing the power needed for their operation.
This electron migration creates an electric current, the lifeblood of any electrical device.
Yo, an electric cell is a baller source of electric current because it’s like a tiny power plant inside your gadgets. Think about it like a hardware component that supplies power to an electrical device , but even cooler. It’s got chemicals inside that get all excited and start flowing like a river of electrons, giving your device the juice it needs to rock and roll.
Simultaneously, ions within the electrolyte solution also participate in this dance of charge. Positive ions, attracted to the negative cathode, migrate towards it, while negative ions, drawn to the positive anode, move in the opposite direction. This ionic movement, coupled with the electron flow, completes the electrical circuit.
An electric cell is a source of electric current because it contains chemicals that react to produce electrons. These electrons flow through a circuit, creating an electric current. In a similar vein, adjusting a truss rod on an electric guitar involves manipulating the tension of the strings , which affects the pitch and intonation of the instrument.
Ultimately, both an electric cell and a properly adjusted truss rod contribute to the generation of sound, albeit in different ways.
Factors Affecting the Electric Current Produced by an Electric Cell
Several factors influence the electric current produced by an electric cell, each playing a crucial role in optimizing its performance. These factors include:
- Electrode Surface Area:The larger the surface area of the electrodes, the more reaction sites are available, leading to increased electron transfer and, consequently, higher current production.
- Electrolyte Concentration:A higher concentration of electrolyte ions enhances the conductivity of the solution, facilitating the movement of ions and boosting current flow.
- Temperature:Elevated temperatures generally increase the kinetic energy of ions and electrons, enhancing their mobility and, thus, increasing the current output of the cell.
Applications of Electric Cells in Everyday Life
Electric cells are ubiquitous in our daily lives, powering a vast array of devices that make our lives easier and more enjoyable. These include:
- Batteries:Portable powerhouses found in everything from watches to laptops, providing reliable and convenient energy on the go.
- Fuel Cells:Environmentally friendly alternatives to traditional batteries, utilizing hydrogen and oxygen to generate electricity with minimal emissions.
- Electroplating:A process that utilizes electric cells to coat objects with a thin layer of metal, enhancing their appearance and durability.
Final Conclusion
In conclusion, electric cells are not just passive components but active sources of electric current, driven by intricate electrochemical processes. Their ability to convert chemical energy into electrical energy has revolutionized our lives, making countless technologies possible. As we continue to explore and refine electric cell technology, we can anticipate even more groundbreaking applications in the future.
FAQ Summary: An Electric Cell Is A Source Of Electric Current Because
What is the basic principle behind an electric cell?
An electric cell harnesses chemical reactions to create a flow of electrons, generating an electric current.
What are the key components of an electric cell?
The anode and cathode are the primary components, responsible for facilitating the movement of ions and electrons.
How does an electric cell produce electricity?
Chemical reactions within the cell cause electrons to flow from the anode to the cathode, creating an electric current.