Atoms with an electric charge are called – Atoms with an electric charge, also known as ions, are fascinating particles that play a crucial role in various scientific fields. From batteries to semiconductors, these charged atoms have unique properties and applications that shape our technological advancements. Let’s delve into the world of atoms with electric charge and explore their intriguing characteristics.
Atoms with an electric charge are called ions, and they can be either positive or negative. For example, an electric utility has determined that a new smart grid technology can help to reduce energy consumption. Ions are also found in many other everyday objects, such as batteries and fluorescent lights.
Atoms with an electric charge can be either positively or negatively charged. Positively charged ions, called cations, are formed when an atom loses one or more electrons. Negatively charged ions, known as anions, are formed when an atom gains one or more electrons.
Atoms with an electric charge are called ions. Just like an electric vehicle starts from rest , ions can also be positive or negative depending on whether they have lost or gained electrons. These charged particles play a crucial role in various chemical reactions and electrical phenomena.
The process of ionization, which creates atoms with electric charge, is a fundamental concept in chemistry and physics.
Atoms with an electric charge are called ions, which can flow through a material to create an electric current. An electrical conductor designed to carry large currents is a material that allows ions to flow easily, making it suitable for use in electrical wiring and other applications where high current flow is required.
Ions are essential for the functioning of electrical circuits, and their ability to flow through conductors is what makes electricity possible.
Definition of Atoms with Electric Charge: Atoms With An Electric Charge Are Called
Atoms with an electric charge are called ions. An ion is an atom that has gained or lost one or more electrons, giving it a net positive or negative charge. The electric charge of an atom is determined by the number of protons and electrons it has.
Atoms with an electric charge are called ions, which can be positive or negative. Ions are formed when an atom gains or loses electrons. For example, an electric turntable 0.750 m in diameter is rotating at a constant rate of 33 1/3 revolutions per minute.
The turntable has a moment of inertia of 0.5 kg m^2. What is the kinetic energy of the turntable?
Protons have a positive charge, while electrons have a negative charge. If an atom has more protons than electrons, it will have a net positive charge. If an atom has more electrons than protons, it will have a net negative charge.
When atoms have an electric charge, they’re known as ions. These charged particles can get even more energized when they enter an electric field. Check out an electron entering an electric field to see how it speeds up and changes direction due to the electric force.
Ions are all around us, playing a crucial role in everything from chemical reactions to the electricity that powers our homes. So, next time you flip on a light switch, remember the tiny charged particles making it all happen!
Ions are formed when atoms gain or lose electrons through chemical reactions.
Atoms with an electric charge are called ions, and they’re all around us. For instance, an operating electric heater draws a current because of the movement of ions. So, next time you’re using an electric heater, remember that it’s all thanks to the movement of ions!
Types of Atoms with Electric Charge
There are two types of atoms with electric charges: cations and anions. Cations are atoms that have lost one or more electrons, giving them a net positive charge. Anions are atoms that have gained one or more electrons, giving them a net negative charge.
Atoms with an electric charge are called ions. Like the an electric refrigerator rated 400w that keeps your food fresh, ions are essential for maintaining the balance of electric charge in the universe. They play a vital role in chemical reactions, electricity, and even the functioning of our bodies.
Understanding ions is crucial for comprehending the world around us.
The process of ionization is the process by which atoms gain or lose electrons. Ionization can occur through a variety of mechanisms, including chemical reactions, heat, and radiation.
Atoms with an electric charge are called ions, and they’re the building blocks of everything around us. From the air we breathe to the electricity that powers our homes, ions are essential to life as we know it. An electric utility is considering a new power plant that would use ion-exchange technology to generate electricity.
This technology has the potential to be more efficient and environmentally friendly than traditional power plants, and it could help us meet our growing energy needs.
Cations
- Positively charged ions
- Formed when an atom loses one or more electrons
- Common cations include Na+, Ca2+, and Fe3+
Anions, Atoms with an electric charge are called
- Negatively charged ions
- Formed when an atom gains one or more electrons
- Common anions include Cl-, O2-, and SO42-
Properties of Atoms with Electric Charge
Atoms with electric charges have unique properties that make them useful in a variety of applications. One of the most important properties of atoms with electric charges is their ability to conduct electricity. This property makes them useful in a variety of electrical devices, such as batteries, capacitors, and semiconductors.
Atoms with electric charges also have a strong tendency to react chemically with other atoms. This property makes them useful in a variety of chemical processes, such as the production of fertilizers and pharmaceuticals.
Applications of Atoms with Electric Charge
Atoms with electric charges are used in a wide variety of applications, including:
Batteries
- Batteries store electrical energy in the form of chemical energy.
- Batteries use atoms with electric charges to create a flow of electrons, which generates electricity.
Capacitors
- Capacitors store electrical energy in the form of an electric field.
- Capacitors use atoms with electric charges to create an electric field, which stores energy.
Semiconductors
- Semiconductors are materials that can conduct electricity under certain conditions.
- Semiconductors use atoms with electric charges to control the flow of electricity.
Conclusion
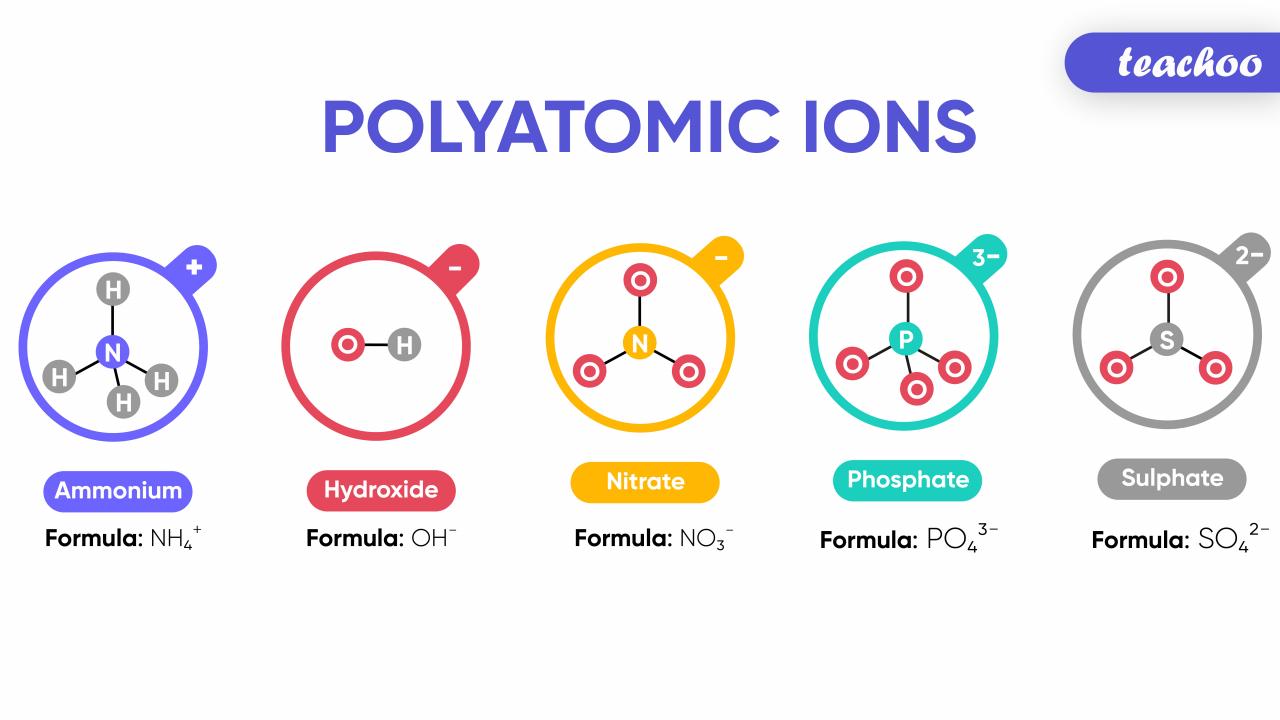
Atoms with electric charge are essential components of our universe and have a wide range of applications in various fields. Understanding their properties and behavior is crucial for advancing scientific research and technological innovations. As we continue to explore the realm of charged atoms, we can expect to uncover even more fascinating discoveries and harness their potential for the benefit of humanity.
FAQs
What are atoms with an electric charge?
Atoms with an electric charge, also known as ions, are atoms that have lost or gained electrons, resulting in a net positive or negative charge.
How are atoms with electric charge formed?
Atoms with an electric charge are called ions, which can be positive or negative. This concept is used in electric motors, which draw a significant amount of current. For instance, an electric motor draws 150 amperes , which is a substantial amount of electricity.
Understanding ions and their behavior is crucial in the design and operation of electric motors.
Atoms with electric charge are formed through the process of ionization, which involves the loss or gain of electrons.
What is the difference between cations and anions?
Cations are positively charged ions formed when an atom loses electrons, while anions are negatively charged ions formed when an atom gains electrons.