An adiabatic system is a closed system that exchanges no heat with its surroundings. This unique property makes it a fascinating concept in thermodynamics and has led to its widespread applications in various fields. Join us as we delve into the world of adiabatic systems, exploring their characteristics, applications, and limitations.
An adiabatic system, like a perfectly insulated thermos, keeps the heat inside. Think of it like a system for heating water from an inlet temperature. This system lets you control the water temperature, just like an adiabatic system controls heat transfer.
So, whether you’re sipping hot coffee or keeping a cool drink icy, adiabatic systems are like the secret sauce that keeps your temperature just right.
Adiabatic systems play a crucial role in understanding energy exchange and conversion. They provide insights into how energy is transferred and utilized within a system without external heat exchange. As we progress, we will uncover the intriguing applications of adiabatic systems in refrigeration, power generation, and industrial processes.
An adiabatic system, where no heat is exchanged with the surroundings, can be compared to an information system’s security, as defined by Charles Pfleeger. According to Charles Pfleeger, an information system is secure when it maintains confidentiality, integrity, and availability.
Just as an adiabatic system remains isolated, a secure information system should protect its data from unauthorized access and ensure its integrity and usability.
Adiabatic Systems
Adiabatic systems are closed systems that do not exchange heat with their surroundings. This means that the total energy of the system remains constant, and any changes in internal energy are due to work done on or by the system.
An adiabatic system is one that does not exchange heat with its surroundings. This means that the total energy of the system remains constant. One way to add energy to an adiabatic system is to add solar panels to an existing system.
Adding solar panels will increase the amount of energy available to the system, which can be used to power devices or charge batteries. An adiabatic system can be used to store energy for later use, or it can be used to generate electricity.
Adiabatic systems are often used in thermodynamics, engineering, and other fields.
An adiabatic system, like a perfectly sealed thermos, is a reminder that some systems are best left alone. Implementing an ERP system can also be a balancing act of advantages and disadvantages. Learn about the pros and cons to avoid a meltdown.
An adiabatic system, on the other hand, teaches us that isolation can sometimes be a virtue.
Characteristics of Adiabatic Systems
Adiabatic systems have several characteristic properties:
- No heat transfer: Adiabatic systems do not exchange heat with their surroundings, meaning the total energy of the system remains constant.
- Constant internal energy: The internal energy of an adiabatic system remains constant, as there is no heat transfer to or from the system.
- Work done: Changes in internal energy are due to work done on or by the system.
Examples of Adiabatic Systems, An adiabatic system
Adiabatic systems are found in various real-world applications:
- Insulated containers: Vacuum flasks and coolers are designed to be adiabatic, minimizing heat transfer and maintaining the temperature of their contents.
- Thermodynamic engines: Adiabatic processes play a crucial role in heat engines, such as diesel engines and gas turbines, where heat is converted into mechanical work.
- Adiabatic calorimetry: Adiabatic calorimeters are used to measure the heat capacity of substances without heat loss to the surroundings.
Thermodynamics of Adiabatic Systems
The first law of thermodynamics applies to adiabatic systems as follows:
dQ = dU + dW
where dQ is the heat transferred, dU is the change in internal energy, and dW is the work done.
In adiabatic systems, dQ = 0, so:
dU =
dW
This equation shows that the change in internal energy is equal to the negative of the work done on the system.
An adiabatic system is like a super chill party where nothing gets in or out. It’s totally isolated, man. But hey, you know what’s even more awesome? Checking out the advantages and disadvantages of an ERP system . It’s like a party planner that helps you keep track of all the cool stuff, from your inventory to your sales.
Plus, it’s like an adiabatic system, keeping your business data safe and sound. Back to our adiabatic system party, it’s all about keeping the good vibes flowing, just like an ERP system keeps your business running smoothly.
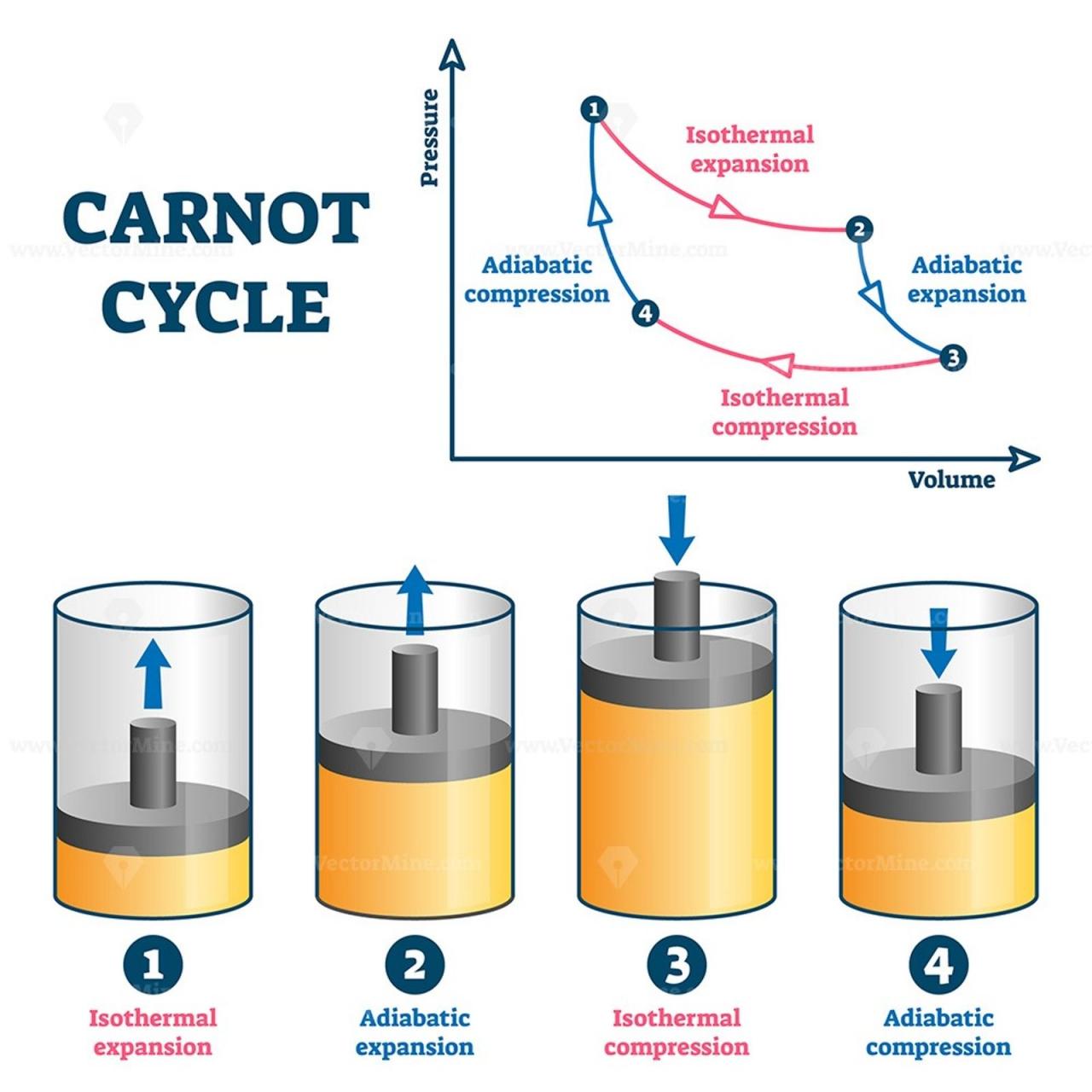
Adiabatic Expansion and Compression
Adiabatic expansion occurs when a gas expands without heat transfer, causing a decrease in temperature and an increase in volume. Conversely, adiabatic compression occurs when a gas is compressed without heat transfer, resulting in an increase in temperature and a decrease in volume.
An adiabatic system, where no heat is exchanged with the surroundings, can be analyzed using a systems approach. Just like a systems analysis of an ecosystem could involve examining energy flows and interactions between components, an adiabatic system can be broken down into its constituent parts and their relationships to understand its overall behavior.
Adiabatic processes are often represented on a pressure-volume (P-V) diagram as curves called adiabats. Adiabats are steeper than isotherms (lines of constant temperature) because the temperature changes during adiabatic processes.
Applications of Adiabatic Systems
Adiabatic systems have numerous applications in various fields:
- Refrigeration and air conditioning: Adiabatic cooling is used in refrigerators and air conditioners to remove heat from a space without using electricity.
- Power generation: Adiabatic turbines are used in power plants to convert heat into mechanical energy, which is then used to generate electricity.
- Industrial processes: Adiabatic processes are employed in various industrial processes, such as the production of plastics and chemicals.
Adiabatic Processes in Different Systems
Adiabatic processes can occur in different systems, including:
- Ideal gases: Adiabatic processes in ideal gases follow the relationship PV^γ = constant, where γ is the specific heat ratio.
- Real gases: Adiabatic processes in real gases deviate from the ideal gas law due to intermolecular forces.
- Solids and liquids: Adiabatic processes in solids and liquids typically involve changes in temperature and density.
Limitations and Considerations
Adiabatic systems have certain limitations and considerations:
- Non-adiabatic effects: In real-world applications, adiabatic systems are often not perfectly adiabatic, and some heat transfer may occur.
- Specific heat capacity: The specific heat capacity of a substance affects the temperature change during adiabatic processes.
- Assumptions: Adiabatic processes assume no heat transfer, which may not always be the case in practice.
Closing Summary
Our exploration of adiabatic systems has shed light on their significance in thermodynamics and their practical applications. These systems offer a unique perspective on energy exchange and have proven valuable in various industries. Understanding the principles of adiabatic processes allows us to optimize energy efficiency and harness the power of thermodynamics for sustainable and innovative solutions.
In the world of thermodynamics, an adiabatic system is like a sealed vault that doesn’t let heat escape or enter. It’s pretty cool, but you know what else is cool? Operating systems! They’re like the brains of your computer, managing resources and running three main functions . And just like an adiabatic system, they keep your computer running smoothly and efficiently, without any heat or fuss.
Frequently Asked Questions: An Adiabatic System
What is the key characteristic of an adiabatic system?
An adiabatic system is characterized by its inability to exchange heat with its surroundings.
How are adiabatic systems used in refrigeration?
Adiabatic expansion is utilized in refrigeration systems to achieve cooling.
What is the relationship between heat, work, and internal energy in an adiabatic system?
In an adiabatic system, the change in internal energy is equal to the work done on or by the system.